What is Liraglutide?
Liraglutide, which if approved, will be marketed under the brand-name "Victoza", is a long-acting glucagon-like peptide-1 (GLP-1) analog that is being developed by Novo Nordisk for the treatment of type 2 diabetes. Liraglutide has a half-life after subcutaneous injection of 11–15 hours, making it suitable for once-daily dosing (in contrast to Byetta's twice daily).
Liraglutide. Image source: Wikipedia, public domain.
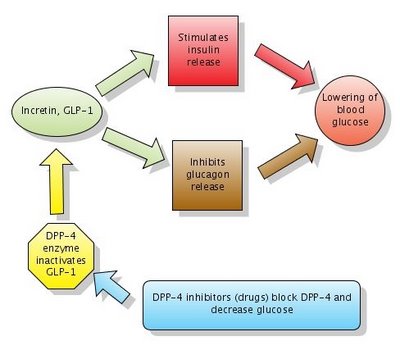
Figure 1. Action of DPP-4 inhibitors. Note that DPP-4 normally inactivates GLP-1. DPP-4 inhibitors block DPP-4 which in turn leaves GLP-1 active.
Click to enlarge the figure. Created with Gliffy.
What is Glucagon-like peptide-1 (GLP-1)?
Glucagon-like peptide-1 (GLP-1) is a GI peptide that stimulates insulin secretion (similar to sulfonylureas). GLP-1 also inhibits glucagon release, gastric emptying and food absorption. GLP-1 and another similar peptide are called incretins. As noted above, incretins have a dual action which leads to lowering blood glucose:
1. Stimulate insulin release
2. Inhibit glucagon release
Exenatide (Byetta) is a GLP-1 receptor agonist approved for adjunctive therapy for patients with DM 2 who are not well controlled on oral agents. It is available only as injections and has to be administered twice daily.
DPP-4 inhibitors or gliptins
Two new medications increase GLP-1 levels by blocking the enzyme which inactivates GLP-1. The enzyme is called DPP-4 (dipeptidyl peptidase-4) and the new medications are called DPP-4 inhibitors or gliptins.
They act similarly to Byetta (see figure above) but have the big advantage to be available in oral form (pills). These 2 new medications for treatment of DM2 are:
- Sitagliptin (Januvia) is taken once a day and it costs about $ 4.50 per pill
- Vildagliptin (Galvus) is waiting for FDA approval but it is already speculated to be at a competitive disadvantage to Januvia because it has to be taken twice a day
New Liraglutide Study
In a double-blind, double-dummy, active-control, parallel-group study, 746 patients with early type 2 diabetes were randomly assigned to once daily liraglutide or glimepiride 8 mg for 1 year.
HbA1c decreased by 0·51% with glimepiride, compared with 0·84% with liraglutide 1·2 mg and 1·14% with liraglutide 1·8 mg. Six patients in the liraglutide groups discontinued treatment because of vomiting.
The authors conclude that liraglutide is safe and effective as initial pharmacological therapy for type 2 diabetes mellitus and leads to greater reductions in HbA1c, weight, hypoglycaemia, and blood pressure than does glimepiride.
References:
Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. The Lancet, Volume 373, Issue 9662, Pages 473 - 481, 7 February 2009.
Liraglutide, from Wikipedia, the free encyclopedia.
DPP-4 Inhibitors for Treatment of Diabetes
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.